Abstract

Introduction: Axi-cel, an autologous anti-CD19 CAR T-cell therapy, is approved for the treatment of adults with follicular lymphoma (FL) after ≥2 lines of prior therapy. ZUMA-5 is a Phase 2, multicenter, single-arm study of axi-cel in patients with R/R iNHL (FL and marginal zone lymphoma [MZL]). In the 2-year analysis of ZUMA-5, the overall response rates (ORR) in patients with FL and MZL were 94% (79% complete response [CR] rate) and 83% (63% CR rate), respectively (Neelapu et al. ASH 2021. Abstract 93). Here, we report updated clinical and pharmacologic outcomes from ZUMA-5 after >3 years median follow-up.
Methods: Eligible patients had R/R FL or MZL after ≥2 lines of therapy (including an anti-CD20 mAb plus an alkylating agent). At enrollment, patients underwent leukapheresis, followed by conditioning chemotherapy and a single axi-cel infusion (2×106 CAR T cells/kg). The primary endpoint was ORR. Time-to-event endpoints were assessed by investigators in all enrolled patients. Exploratory analyses of lymphoma-specific survival were performed, where deaths unrelated to progression, axi-cel, or conditioning chemotherapy were not considered events of interest. Univariable and multivariable analyses were conducted using random forest analysis to rank the association of pharmacologic covariates with efficacy and toxicity.
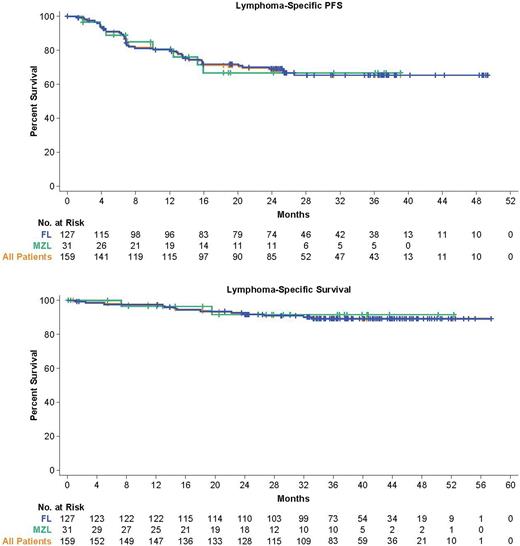
Results: A total of 159 patients were enrolled (127 FL; 31 MZL) and 152 were treated with axi-cel (124 FL; 28 MZL). As of March 31, 2022, the median follow-up in enrolled patients was 40.5 months (range, 8.3-57.4; FL: 41.7, MZL: 31.8). ORR and CR rates were largely similar to the 2-year analysis. In all enrolled patients, median duration of response (DOR) was 38.6 months (FL: 38.6, MZL: not reached [NR]). Median DOR was NR in patients with a CR and was 4.9 months in those with a partial response. Median progression-free survival (PFS) was 40.2 months (FL: 40.2, MZL: NR). Median PFS among patients with FL with (n=70) or without (n=41) progression <2 years after initial chemoimmunotherapy (POD24) was 40.2 months and NR, respectively. Estimated 36-month PFS was largely consistent in all patients with iNHL, regardless of other high-risk characteristics, including ≥3 prior lines of therapy and double-refractory disease. Medians of time to next treatment and overall survival (OS) were not reached; 36-month OS rate was 75%. Medians of lymphoma-specific PFS and lymphoma-specific survival were not reached; 36-month rates were 65% and 89%, respectively.
Grade ≥3 adverse events (AEs) of interest occurring among treated patients since the 2-year analysis were largely in recently enrolled patients with MZL, including neurologic events in 1, cytopenias in 4, and infections in 2 (1 in FL). Since the 2-year analysis, 10 additional patients died due to progression (n=1), AEs (n=3; none related to axi-cel), and other causes (n=6).
Among treated patients, peak CAR T-cell levels were higher in those with ongoing responses at 36 months (53.9 cells/µL) than those who relapsed (29.6 cells/µL) or nonresponders (22.2 cells/µL). In patients with FL, peak levels of circulating CAR T cells normalized to baseline tumor burden in conjunction with elevated preinfusion inflammatory markers and regulatory T-cell (Treg)-related chemokines associated with relapse. Multivariable analyses in patients with FL, to be detailed in the presentation, further identified key covariates that differentially associated with efficacy and toxicity.
Conclusions: After 3 years of follow-up in ZUMA-5, axi-cel demonstrated continued durable responses in patients with R/R iNHL, with improved survival observed in patients with MZL. Late progression or death due to lymphoma or study treatment were uncommon and no new safety signals arose since the 2-year analysis. Preinfusion immunosuppressive Treg-related biomarkers associated with relapse in patients with FL.
Disclosures
Neelapu:Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Pfizer: Consultancy, Honoraria, Other: Personal fees; Bluebird Bio: Consultancy, Honoraria; Bio Ascend: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Calibr: Consultancy, Honoraria, Other: Personal fees; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Acerta: Research Funding; Cellectis: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy.; Poseida: Research Funding; Karus Therapeutics: Research Funding; Medscape: Consultancy, Honoraria; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Aptitude Health: Consultancy, Research Funding; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees. Chavez:Astrazeneca: Research Funding; Epyzime: Honoraria; Janssen: Research Funding; Merck: Research Funding; BeiGene: Honoraria; TG Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; ADC Therapeutics: Research Funding; AdiCet: Consultancy; GenMab: Consultancy. Sehgal:Juno: Other: Grants, Research Funding; Milltenyiz: Research Funding; Kite, a Gilead Compnay: Other: Grants, Research Funding; OncLive: Honoraria; PeerView: Honoraria. Epperla:Incyte: Speakers Bureau; Novartis: Honoraria; TG Therapeutics: Other: Ad Board; BeiGene: Other: Ad Board; Seattle Genetics: Other: Ad Board; Pharmacyclics: Other: Ad Board. Ulrickson:Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bachy:Kite, a Gilead Company: Honoraria. Munshi:Kite, a Gilead Company: Speakers Bureau; Incyte: Speakers Bureau; Amgen: Current holder of stock options in a privately-held company. Casulo:Verastem: Research Funding; Secura Bio: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Bristol Myers Squibb: Research Funding. Maloney:A2 Biotherapeutics: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Other: Data Safety Monitory Board , Research Funding; Genentech: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Legand Biotech: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Navan Technologies: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Umoja: Consultancy, Honoraria; Bioline Rx: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitory Board ; Fred Hutch: Patents & Royalties: For Patients licensed to Juno ; A2 Biotherapeutics: Current holder of stock options in a privately-held company. de Vos:BeiGene: Other: Participation on a Data Safety Advisory Board. Reshef:University Of Pennsylvania: Other: Data Safety Monitoring Board; Capstan Therapeutics: Consultancy; BMS: Honoraria; Precision Biosciences: Research Funding; Incyte: Research Funding; Gilead: Consultancy, Honoraria, Other: Travel Support, Research Funding; Novartis: Honoraria; Takeda: Research Funding; Jasper: Consultancy; Synthekine: Consultancy; Atara Biotherapeutics: Consultancy, Research Funding; Pharmacyclics: Research Funding; Immatics: Research Funding; Bayer: Consultancy; MidaTech: Consultancy; Regeneron: Consultancy; TScan: Consultancy; Shire: Research Funding; J&J: Research Funding. Leslie:Eli Lily: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Speakers Bureau; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Jansssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Celegene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support. Oluwole:Janssen: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; TG Therapeutics: Consultancy; Curio Science: Consultancy; ADC Therapeutics: Consultancy; Kite, a Gilead Company: Research Funding. Yakoub-Agha:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria; Janssen: Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support. Rosenblatt:University of Miami Miller School of Medicine: Patents & Royalties; Biograph 55: Research Funding; Synergys: Other. Yan:Kite, a Gilead Company: Current Employment. Song:Gilead Sciences: Current holder of stock options in a privately-held company, Patents & Royalties; Kite, a Gilead Company: Current Employment, Patents & Royalties: Gilead. Peng:Kite, a Gilead Company: Current Employment. Lui:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other: Travel support. Wulff:Kite, a Gilead Company: Current Employment. Shen:Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties; Atara: Patents & Royalties. Poddar:UCLA: Other: intellectual Property, Patents & Royalties; Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company. Miao:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Beygi:Kite, a Gilead Company: Current Employment, Research Funding. Jacobson:Ispen: Consultancy, Honoraria; ImmPACT Bio: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Celgene: Other: Travel Support; Axis: Speakers Bureau; Pfizer: Other: Travel Support, Research Funding; Lonza: Consultancy, Honoraria, Other: Travel Support; Instil Bio: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Humanigen: Consultancy, Honoraria, Other: Travel Support; Epizyme: Consultancy, Honoraria; Nkarta: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; Clinical Care Options: Speakers Bureau; Precision BioSciences: Consultancy, Honoraria, Other: Travel Support; Novartis: Consultancy, Honoraria, Other: Travel Support; BMS/Celgene: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal